
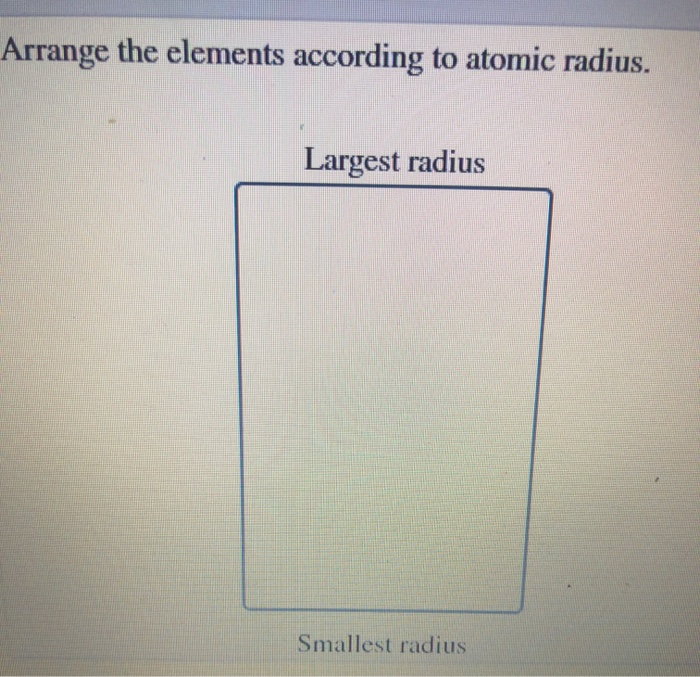
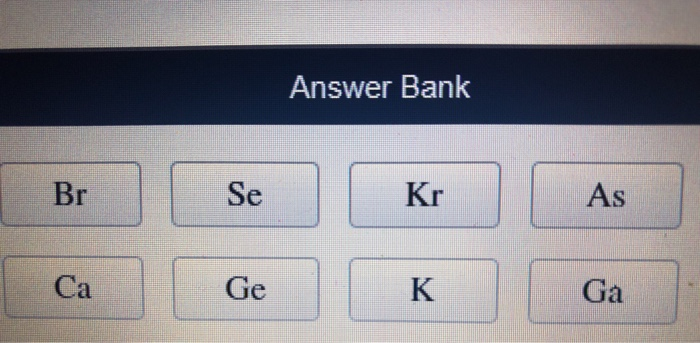
Note: It is possible for a vast majority of elements to form covalent molecules in which two like atoms are held together by a single covalent bond. Thus, we can conclude that the answer to this question is option (A). > Atomic radii decrease with increase in atomic number because within the same period the number of shells of electrons do not increase but positive charge on nucleus gradually increases. 7.48 Arrange the elements in each of the following groups in order of increasing. So, fluorine will have the smallest atomic radii among them three and oxygen will have smaller atomic radii than carbon’s. C, O and F have atomic numbers of 6, 8 and 9 respectively. > C, O and F are elements of the same period and in the same period atomic radius decreases with increase in atomic number. So, its atomic radius will be more than second period elements and less than bromine. > Now, chlorine is a third period element and hence it will have a shell of electrons more than the second period elements. Using only the periodic table arrange the following elements in order of increasing atomic radius: argon, helium, xenon, neon Smallest Largest Please answer this question according to the general rules you have learned regarding periodic trends. Solve any question of Classification Of Elements And Periodicity In Properties with:. On moving from right to left in a period, the atomic radius decreases. On moving down the group, the atomic radius increases. So, bromine will have an extra shell of electrons and hence its atomic radius will be biggest amongst all. Rank each of the following in order of INCREASING atomic radius a. The increasing order of atomic radius is Mg
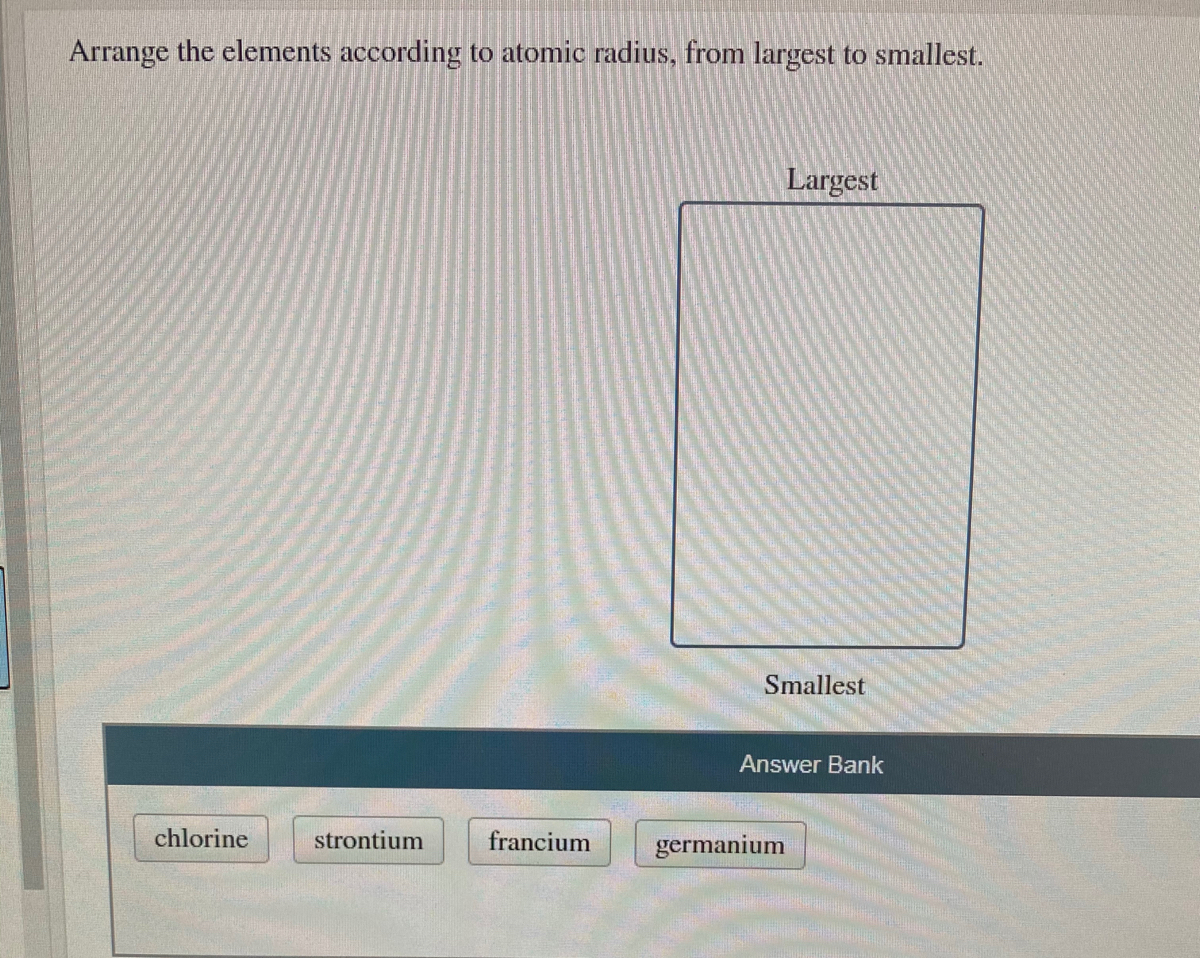
Hint: The atomic radius shows the distance between the centre of the atom and the valence orbital of the atom.


 0 kommentar(er)
0 kommentar(er)
